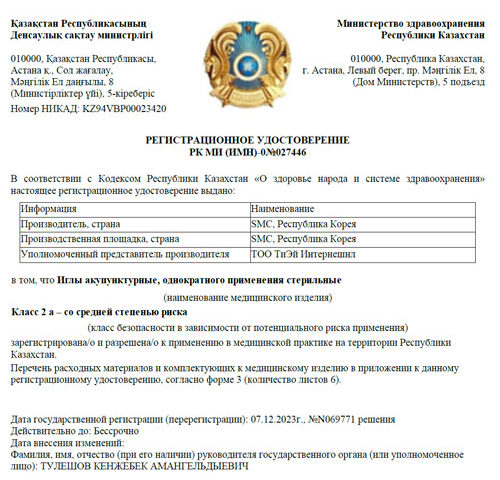
Badim Acupuncture Needles: Success in Kazakhstan
We are delighted to share a momentous achievement for our esteemed brand, Badim, as it has successfully completed registration in Kazakhstan. Accompanied by the official Medical Device Authorization, Badim is now approved for marketing promotion within the region.
This significant milestone underscores our commitment to delivering high-quality healthcare products and ensuring compliance with regulatory standards. We extend our gratitude to our partner, TA International Ltd., for contributing to this success and look forward to further advancements in our global presence.