Zentia
$0.00
PRODUCT INFORMATION
NAME
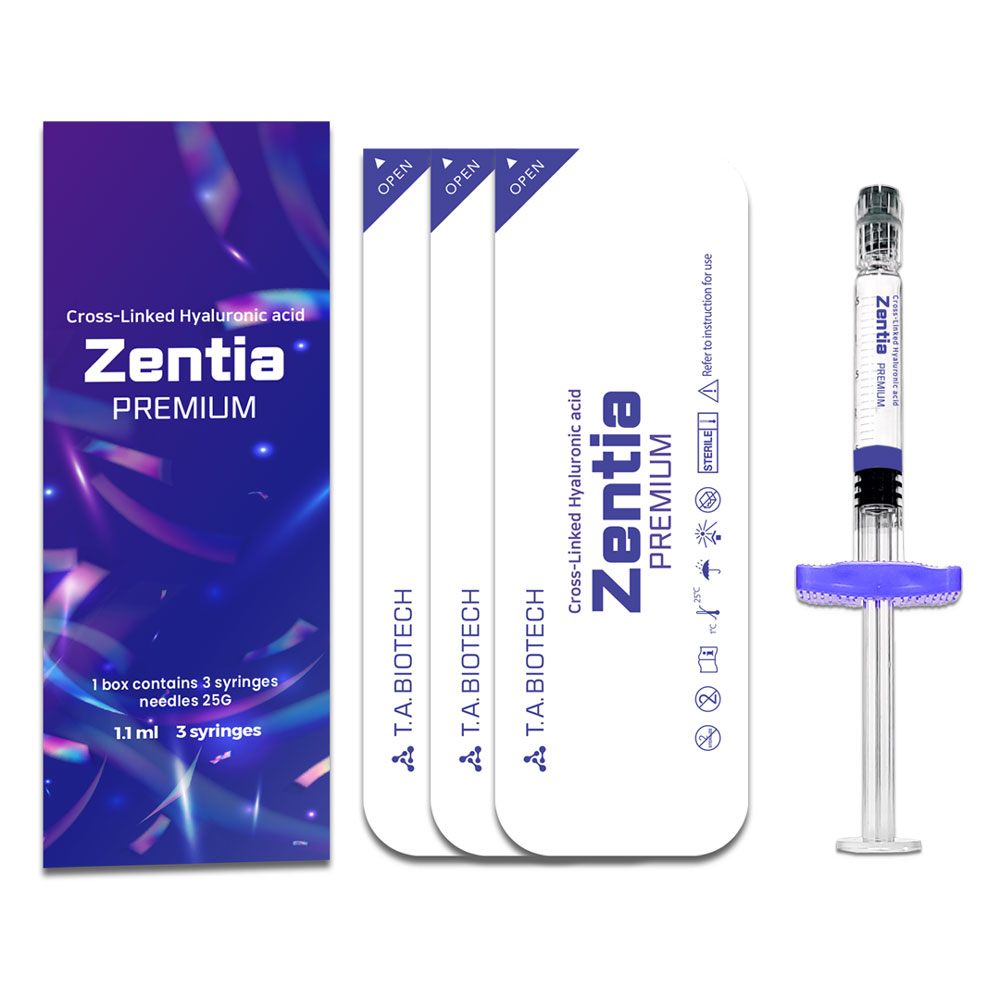
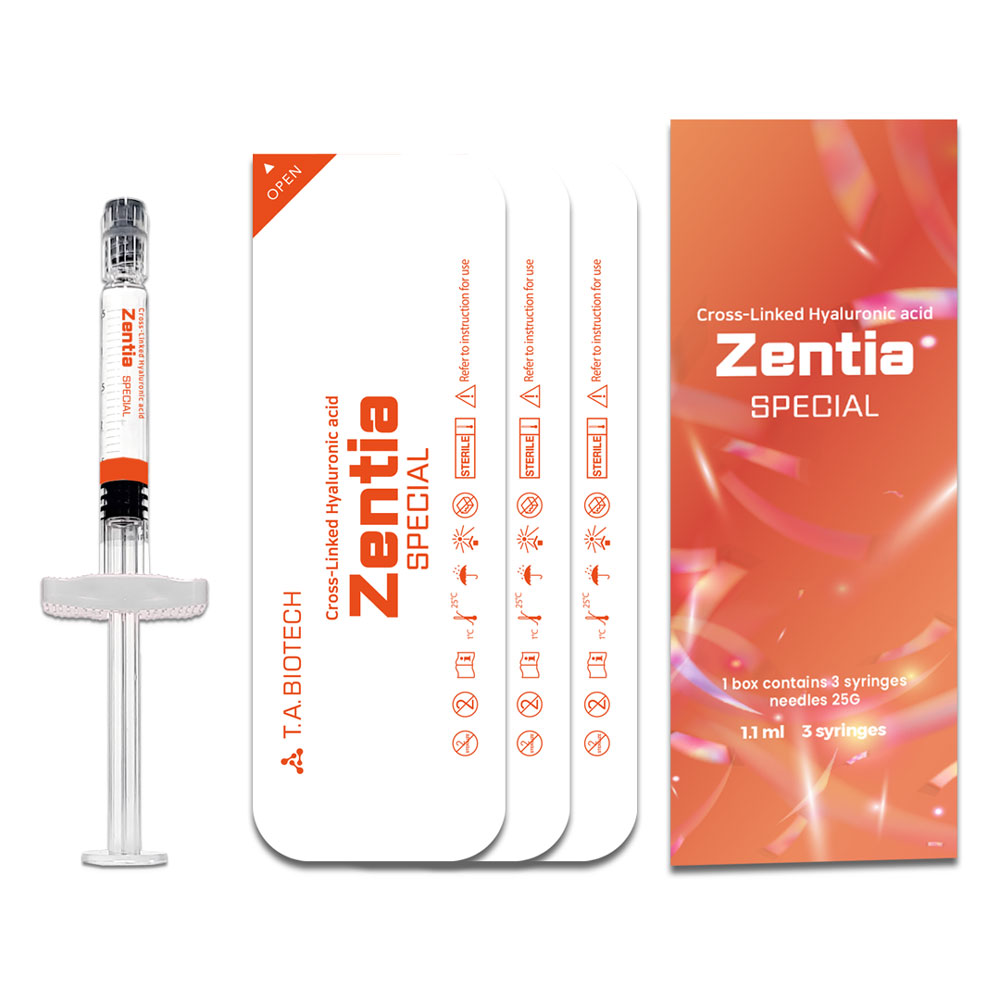
Zentia
Packaging
1.1 ml 3 syringes
How to use
Should be performed by a certified professional.
Storage
Store in 2 – 25°C. Protect from direct sunlight and freezing
VENDOR
T.A Bio Tech Co., Ltd.
PRODUCT INTRODUCTION
Dermal Filler Zentia
Description
Zentia Cross-Linked Hyaluronic Acid
Performance: Hyaluronic acid is a high molecular substance composed of repeated disaccharides of N-acetyl glucosamin and glucuronic acid. Zentia has realized a stable gel through precision cross-linking technology. The stable stucture of hyaluronic acid gel fills the space, supports the structure, protects the cell, replenishes the volume of wrinkled skin and keeps it natural.
Safety: The active ingredient used in Zentia is non-animal. It is a human-adapted component, contains high-purity hyaluronic acid and naturally decomposed over time. Hyaluronic acid cross-linking gel can be corrected through hyaluronidase when a problem occurs. It is safe by providing the level of endotoxin and crosslinking agent below the detection limit through strict manufacturing process management.
Usability: Precisely designed handles and push rods keep the pressure at the time of injection evenly, enabling stable treatment. The monophasic structure is a regular and stable gel structure, and it is a specialized technology applied to Zentia that realizes natural volume and soft injection feeling.
Pain relief: Zentia contains Lidocaine to improve pain-related discomfort during the treatment.
| Product Name | SPECIAL | PREMIUM |
|---|---|---|
| Injection Site | Mid to deep dermis | Mid to deep dermis subcutaneous layer |
| HA Concentration | 24 mg / ml | |
| Visco-elasticity | Mid | High |
| Duration | 9-18 months | 12-24 months |
| Packaging unit | 27G*2 | 25G*2 |
| Injection Area | Nasolabial folds, forehead, marionette lines and face lifting | Facial contouring, chin and chick augmentation |
| Syringe Volume | 1.1ml * 3syr | |
| Lidocaine | 0.3% | |
| Shelf Life | 24 months | |
| Storage | Store in 2 – 25°C. Protect from direct sunlight and freezing | |
The durations are depends on age, lifestyle and the skill of a practitioner.
Cross-Linked Hyaluronic acid Zentia
High Quality Raw materials
Zentia is manufactured using 100% authentic high-quality hyaluronic acid, every Finished product complies with European Pharmacopoeia on the endotoxin level.
Endotoxin – Test items. Acceptance Criteria – Not more than 12.5 EU/ml. Results – Less than 0.5 EU/ml
Purification Process High
10 steps only Zentia’s unique manufacturing technology enables high elasticity with lower amount of cross-linking agent and residual BDDE
BDDE – Test items. Acceptance Criteria – <2ppm (NMT 2ppm). Results – Not detected
Elasticity Cohesiveness High
No swelling, no break during injection, maintaining original shape and natural look. Keep shape after injection. Not easily broken by water
Viscosity (Apparent viscosity, 50Hz) – Test items. Acceptance Criteria – 13100cp~ 17000cp. Results – 16000cp
High viscoelasticity and cohesiveness, but very comfortable injection force
Test items – Extrusion force. Acceptance Criteria – Not more than 25N. Results – 10N
Dermal Filler Zentia
-
Hyaluronic Acid;
-
Dermal Filler;
-
24mg/ml;
-
Lidocaine 3mg/ml;
-
High HA concentration;
-
Longer Duration;
-
Safety Raw materials;
-
No residual BDDE;
-
High Elasticity.
Cross-Linked Hyaluronic acid Zentia SPECIAL 1.1 ml 3 syringes
-
Forehead Augmentation
-
Nosolabial folds
-
Cheek
Filler Zentia PREMIUM 1.1 ml 3 syringes
-
Rhinoplasty
-
Chin augmentation
-
Antero-medial malar
DETAILED
You may also like…
-
Add to cart
Zentia PREMIUM
$0.00 -
Add to cart
Zentia SPECIAL
$0.00 -
Add to cart
POLYBOOST CL
$0.00














Reviews
There are no reviews yet.